Malaysian
Journal of Analytical Sciences Vol 26 No 2
(2022): 229 - 240
THE CHEMICAL PROPERTIES AND ANTI-ACNE ACTIVITY
DETERMINATION of Swietenia macrophylla SEED EXTRACTS
(Penentuan Ciri Kimia dan Aktiviti
Anti-Jerawat Ekstrak Biji Swietenia
Macrophylla)
Non Daina Masdar1, Noor
Hafizah Uyup1, Zamzila Erdawati Zainol2, Muhammad Akmal
Roslani2, Siti Nur Syarifa Anuar1,
Muhamad Azhar Zulkafle3*
1Department of Chemistry
2Department of Marine
3Department of Biology
Faculty of Applied Sciences,
Universiti Teknologi MARA, Perlis Branch, Arau Campus,
02600 Arau, Perlis, Malaysia
*Corresponding author:
azharz@uitm.edu.my
Received: 7 October 2021; Accepted: 18 December 2021; Published: 28 April 2022
Abstract
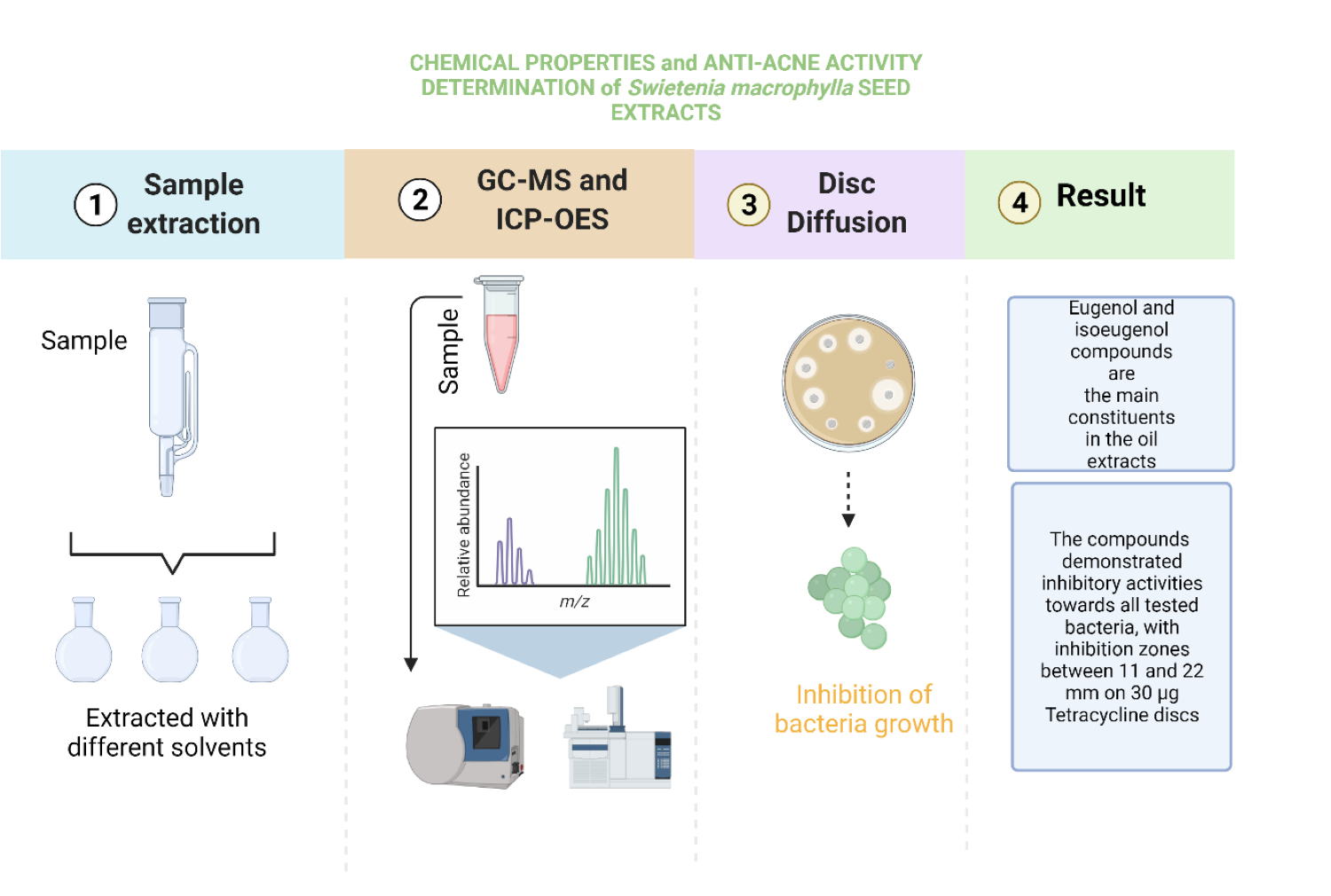
Acne is a common skin disorder usually treated using
antibiotics and drugs. However, until today, dermatologists struggle to treat
acne resistance towards topical treatment over a long period. One of the
solutions is using natural bioactive compounds from plant extracts. In this
work, Swietenia macrophylla seeds oil, rich in active compounds, was
used to inhibit acne-causing bacteria, i.e., Propionibacterium acnes,
Staphylococcus aureus, and Staphylococcus epidermidis. The seed
powder sample was extracted using the Soxhlet extraction method in three
different solvents for six hours and ten cycles. The seed extracts were
analysed using a gas chromatography-mass spectrophotometer (GC-MS), and a disc
diffusion assay was performed to analyse the antibacterial activities. The
heavy metal analysis was determined by inductively coupled plasma atomic
emission spectroscopy (ICP-OES). The results show that bioactive compound
yields are 37-72%. Eugenol and isoeugenol compounds are the main constituents
in the oil extracts with 98% and 97% quality. The compounds also demonstrated
inhibitory activities towards all tested bacteria, with inhibition zones
between 11 and 22 mm on 30 ?g tetracycline discs. These compounds without
isolation work also showed inhibitory activity against all bacteria tested with
inhibition zones ranging from 11 to 21 mm.
Keywords: Swietenia
macrophylla, acne, natural treatment, Propionibacterium
acne, Staphylococcus epidermidis
Abstrak
Jerawat
adalah penyakit kulit biasa dan selalunya dirawat menggunakan antibiotik
dan ubat-ubatan. Namun, sehingga hari ini, pakar dermatologi masih belum dapat
merawat ketahanan jerawat terhadap rawatan
permukaan dalam jangka masa panjang. Salah satu penyelesaiannya adalah
menggunakan sebatian bioaktif semula jadi dari ekstrak tumbuhan. Dalam kajian
ini, minyak biji Swietenia macrophylla yang kaya dengan sebatian aktif,
digunakan untuk merencat bakteria penyebab jerawat iaitu Propionibacterium
acnes, Staphylococcus aureus, dan Staphylococcus
epidermidis. Sampel serbuk biji diekstrak menggunakan kaedah pengekstrakan
Soxhlet dalam tiga jenis pelarut berbeza selama enam jam dan sepuluh kitaran.
Analisis hasil ekstrak dijalankan menggunakan kromatografi gas-spektrofotometer
jisim (GC-MS) dan asai cakera resapan dilakukan untuk menganalisis aktiviti
antibakteria. Analisis logam berat pula ditentukan melalui spektrometri
pancaran optikal-plasma gandingan aruhan (ICP-OES). Hasil kajian menunjukkan
bahawa hasil sebatian bioaktif ialah 37-72%. Sebatian eugenol dan isoeugenol
merupakan sebatian utama dalam sampel ekstrak minyak dengan kualiti 98% dan
97%. Sebatian tanpa kerja pengasingan ini
juga menunjukkan aktiviti perencatan terhadap semua bakteria yang diuji
dengan zon perencatan berjulat antara 11 hingga 21 mm.
Kata kunci: Swietenia
macrophylla,
jerawat, rawatan semula jadi, Propionibacterium
acne, Staphylococcus epidermidis
Graphical Abstract
References
1.
Zaghloul, S. S., Cunliffe, W.
J. and Goodfield, M. J. D. (2005). Objective assessment of compliance with
treatments in acne. British Journal of Dermatology, 152(5): 1015-1021.
2.
Fox, L., Csongradi, C., Aucamp,
M., Du Plessis, J. and Gerber, M. (2016). Treatment modalities for acne. Molecules,
21(8): 1-20.
3.
Savage, A. E. O. (1985). United
States Patent, 19(54): pp. 19.
4.
Vora, J., Srivastava, A. and
Modi, H. (2018). Antibacterial and antioxidant strategies for acne treatment
through plant extracts. Informatics in Medicine Unlocked,
13: 128-132.
5.
Afsar, Z. and Khanama, S. (2016). Formulation and evaluation of polyherbal soap and
sanitiser. International Research Journal of Pharmacy, 7(8): 54-46.
6.
Platsidaki E. and Dessinioti C.
(2018). Recent advances in understanding Propionibacterium acnes (Cutibacterium
acnes) in acne. F1000Research, 7(F1000 Faculty Rev:1953).
7.
Tan, A. U., Schlosser B. J. and
Paller, A.S. (2017). A review of diagnosis and treatment of acne in adult
female patients. International Journal of Women's Dermatology, 4(2):
56-71.
8.
Eid, A. M. M., Elmarzugi, N. A.
and El-Enshasy, H. A. (2013). A review on the phytopharmacological effect of Swietenia
macrophylla. International Journal of Pharmacy and Pharmaceutical
Sciences, 5(3): 47-53.
9.
Suliman, M. B. (2018).
Preliminary phytochemical screening and thin layer chromatography analysis of Swietenia
Macrophylla King methanol extracts. Chemistry of Advanced Materials,
3(1): 1-7.
10.
Durai, M., Balamuniappan, G.
and Geetha, S. (2016). Phytochemical screening and antimicrobial activity of
leaf, seed and central-fruit-axis crude extract of Swietenia macrophylla
King. Journal of Pharmacognosy and Phytochemistry, 181(53): 181-186.
11.
Hashim, M. A., Yam, M. F., Hor,
S. Y., Lim, C. P., Asmawi, M. Z. and Sadikun, A. (2013). Anti-hyperglycaemic
activity of Swietenia macrophylla king (meliaceae) seed extracts in normoglycemic rats undergoing glucose
tolerance tests. Chinese Medicine (United Kingdom), 8(1): 1-8.
12.
Wu, Q., Li, Y., Hu, H., Wang,
M., Wu, Z. and Xu, W. (2012). Rapid identification of Staphylococcus aureus:
FISH versus PCR methods. Laboratory Medicine, 43(6): 276-280.
13.
Naghdi, N. and Ghane, M.
(2017). A comparison of culture and PCR methods for identifying Propionibacterium
acnes in lesions isolated from patients with acne. Turkish Journal of
Medical Sciences, 47(3): 967-972.
14.
Park, J., Lee, J., Jung, E.,
Park, Y., Kim, K., Park, B., Jung, K., Park, E., Kim, J. and Park, D. (2004). In
vitro antibacterial and anti-inflammatory effects of honokiol and magnolol
against Propionibacterium sp. European Journal of Pharmacology,
496(1): 189-195.
15.
Nirmal, N. P. and
Panichayupakaranant, P. (2014). Anti-propionibacterium acnes assay-guided
purification of brazilin and preparation of brazilin rich extract from Caesalpinia
sappan heartwood. Pharmaceutical Biology, 52(9): 1204-1207.
16.
Habib, F., Rind, R., Durani,
N., Latif Bhutto, A., Buriro, R. S., Tunio, A., Aijaz, N., Lakho, S., Ghulam,
B. and Shoaib, M. (2015). Morphological and cultural characterization of Staphylococcus aureus isolated from
different animal species. Journal of Applied, Environmental and Biological
Sciences, 5(2): 15-26.
17.
Shinkafi, S., and Ndanusa, H.
(2013). Antibacterial activity of citrus lemon on acne vulgaris
(pimples). International Journal of Science Inventions Today, 2(5):
397-409.
18.
Do, Q. D., Angkawijaya, A. E.,
Tran-Nguyen, P. L., Huynh, L. H., Soetaredjo, F. E., Ismadji, S. and Ju, Y. H.
(2014). Effect of extraction solvent on total phenol content, total flavonoid
content, and antioxidant activity of Limnophila aromatica. Journal of
Food and Drug Analysis, 22(3): 296-302.
19.
Nawaz, H., Shad M. A., Rehman,
A. N., Andaleeb, H. and Ullah, N. (2020). Effect of solvent polarity on
extraction yield and antioxidant properties of phytochemicals from bean (Phaseolus
vulgaris) seeds. Brazilian Journal of Pharmaceutical Sciences, 56:
17129.
20.
Che Sulaiman I. S. and Basri,
M. (2017). Effects of temperature, time, and solvent ratio on the extraction of
phenolic compounds and the anti-radical activity of Clinacanthus nutans
Lindau leaves by response surface methodology. Chemistry Central Journal,
11(1): 54.
21.
Akbar A., Soekamto N. H.,
Firdaus and Bahrun (2021) Antioxidant of n-hexane, ethyl acetate and methanol
extracts of Padina sp. with DPPH method. Earth and Environmental
Science 800: 012019.
22.
Azhari, Nour, H., Mohammed,
Sulieman, B., Mashitah, Yousf, M. and Adam, M. M. (2016). Bioassay-guided
isolation and identification of antifungal compounds from seeds of Swietenia
macrophylla King. Australian Journal of Basic and Applied Sciences,
10(17): 55-62.
23.
Aquirre-Becerra H.,
Pineda-Noeto S. A., Garcia-trejo J.F., Guevara-Gonzalez R. G., Feregrino-Perez
A. A., Alerez-Mayorga B. L. and Rivera-Pastrana D. M. (2020). Jacaranda flower
(Jacarand mimosifolia) as alternaive for antioxidant and antimicrobial
use. Heliyon, 6(12): e05802.
24.
Eid A., Elmarzugi N. and
El-Enshasy H. (2013). A review on the phytopharmacological effect of Swietenia
macrophylla. International Journal of Pharmacy and Pharmaceutical
Sciences, 5: 47-53.
25.
Moghadamtousi S. Z., Goh B. H.,
Chan C. K., Shabab T. and Kadir, H. A. (2013).?
Biological activities and phytochemicals of Swietenia macrophylla
King. Molecules, 18: 10465-10483.
26.
Sharma S., Kumari A., Dhatwalia
J., Guleria I., Lal S., Upadhyay N., Kumar V. and Kumar A., (2021), Effect of
solvents extraction on phytochemical profile and biological activities of two Ocimum
species: A comparative study. Journal of Applied Research on Medicinal and
Aromatic Plants, 25(2021): 100348.
27.
Koeduka, T. (2014). The
phenylpropene synthase pathway and its applications in the engineering of
volatile phenylpropanoids in plants. Plant
Biotechnology, 2014: 14-0801.
28.
Nejad, S. M., Ozgunes, H.
and Başaran, N. (2017). Pharmacological and toxicological properties of
eugenol. Turkish Journal of
Pharmaceutical Sciences, 14(2): 201.
29.
Bendre, R. S., Rajput, J. D.,
Bagul, S. D. and Karandikar, P. S. (2016). Outlooks on medicinal properties of
eugenol and its synthetic derivatives. Natural
Product Chemistry Research, 4(3): 1-6.
30.
Mustafa, H. S. I. (2014). Staphylococcus
aureus can produce catalase enzyme when adding to human WBCs as a source of
productions in human plasma or serum in the laboratory. Open Journal of
Medical Microbiology, 04: 249251.
31.
Kallstrom, G., Chang, T.,
Albertson, M., Morilla, D., Fisher, M. A. and Eberly, B. (2011). Recovery of a
catalase-negative Staphylococcus epidermidis strain in blood and urine
cultures from a patient with pyelonephritis. Journal of Clinical
Microbiology, 49(11): 4018-4019.
32. Cauich-Sanchez, P., Alatriste-Mondragon, F., Garcia-Cano, E. and Aquino-Santiago, C. (2001). Identification of anaerobic nonsporeforming gram-positive bacilli by biochemical tests and gas-liquid chromatography. Revista Latinoamericana de Microbiologia, 43(1): 27-35.
33. Rolf, L. (2011). Propionibacterium acnes and its phages department of clinical sciences. Department of Clinical Sciences, Lund University: pp. 1-85. Kuntom, A. and Kifli, (1998). Properties of soaps derived from distilled palm stearin and palm kernel fatty acids. Journal of Surfactants and Detergents, 1(3): 329-334.
34.
Kuntom, A. and Kifli, (1998).
Properties of soaps derived from distilled palm stearin and palm kernel fatty
acids. Journal of Surfactants and Detergents, 1(3): 329-334.
35.
Panawala, L. (2017). Difference
between gram positive and gram-negative bacteria. Epedıaa, 3: 1-13.
36.
Tenover F. C. (2019).
Antimicrobial susceptibility testing. Encyclopedia of Microbiology (Fourth
Edition): pp. 166-175.
37.
Rodloff, A., Bauer, T., Ewig,
S., Kujath, P. and Muller, E. (2008). Susceptible, intermediate, and resistant
? the intensity of antibiotic action. Deutsches Aerzteblatt Online,
105(39): 657-662.
38.
Aditi, F. Y., Rahman, S. S. and
Hossain, M. M. (2017). A study on the microbiological status of mineral
drinking water. The Open Microbiology
Journal, 11: 31.
39.
Hepp, N. M., Mindak, W. R.,
Gasper, J. W., Thompson, C. B. and Barrows, J. N. (2014). Survey of cosmetics
for arsenic, cadmium, chromium, cobalt, lead, mercury, and nickel content. Journal
Cosmetic Sciences, 65(3): 125.