Malaysian
Journal of Analytical Sciences Vol 26 No 2
(2022): 347 - 359
THERMAL
DECOMPOSITION OF CALCIUM CARBONATE IN CHICKEN EGGSHELLS: STUDY ON TEMPERATURE
AND CONTACT TIME
(Penguraian Kalsium Karbonat dalam Kulit
Telur Ayam: Kajian Mengenai Suhu dan Masa)
Nadia Razali1, Nurriswin Jumadi1,2*,
Adlin Yasmin Jalani1, Nurhanim Zulaikha Kamarulzaman1, Khairul Faizal Pa'ee3
1Section of Environmental
Engineering Technology
2Section of Process
Engineering Technology
3Section of Food Engineering
Technology
Universiti Kuala Lumpur MICET, 78000 Alor Gajah, Melaka,
Malaysia
4Kolej Komuniti Jelebu,
71600 Kuala Klawang, Negeri Sembilan, Malaysia
*Corresponding
author: nurriswin@s.unikl.edu.my
Received: 13 September 2021; Accepted: 3 February 2022; Published: 28 April 2022
Abstract
Within
the context of a circular economy, the recycling or valorisation of eggshells,
which are typically discarded in landfills, represents an opportunity. The
primary compound in eggshells is calcium carbonate (CaCO3), which
can be decomposed into calcium oxide (CaO) by calcination. This study examined
the calcination conditions (temperature and contact time) for the optimum CaCO3
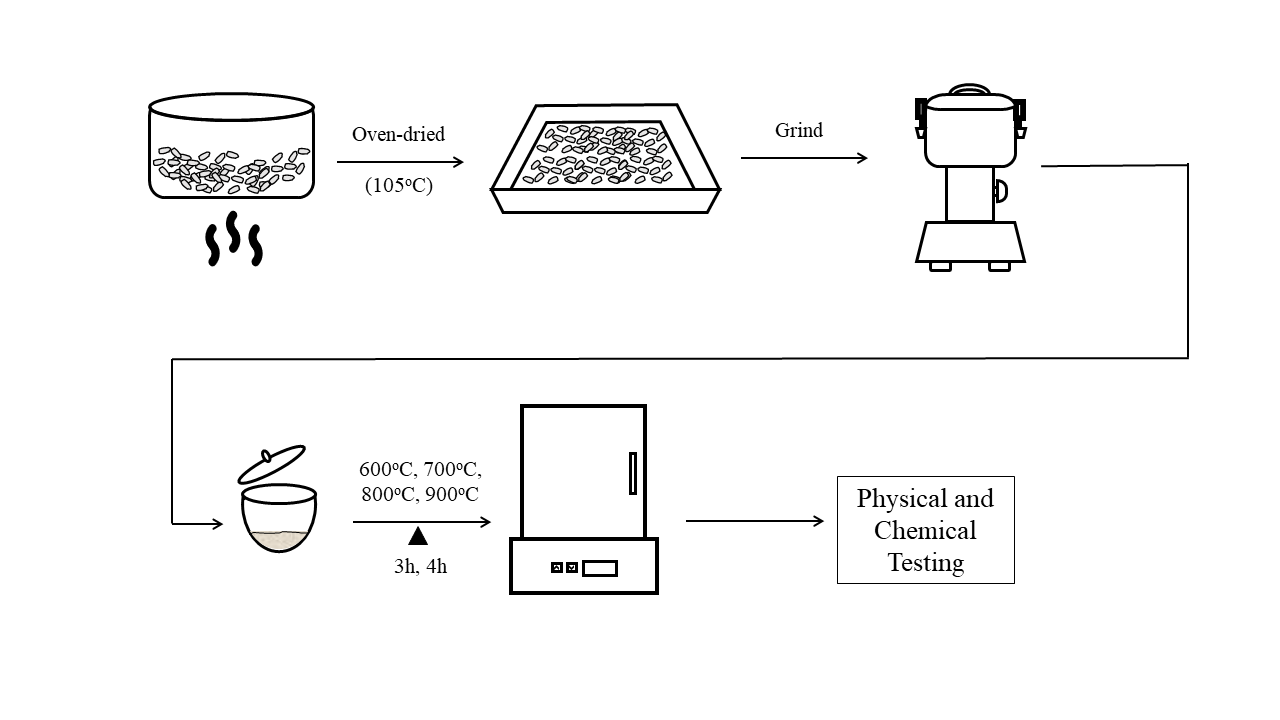
decomposition rate. The eggshell samples were pre-treated to eliminate dirt and
unnecessary biological substance, ground into powder and sieved. The primary
physical and chemical characteristics of eggshell powder were studied,
including colour changes, mass loss, bulk density, moisture content, pH,
thermal properties, and identification of chemical bonds and compounds in a
molecule. This study evaluated the physical and chemical properties of the
synthesised CaCO3 from eggshells, moisture content, bulk density,
pH, FTIR, and XRD. The results showed significant differences in the samples'
colour transition at various temperatures and contact times based on the
physical observation. The TGA analysis showed that eggshell powder decomposed
at a temperature range of 600 - 900 oC. The FTIR results reported that for the
calcine samples, the grey powder consists of CaCO3, while the solid
white powder consists of metal oxide content. Similar seven diffraction peaks
were observed in the XRD analysis for calcination at 900 oC and industrial CaO
(32.25, 37.41, 53.92, 64.18, 67.41, 79.70, and 88.58). The eggshell powder
calcined at the temperature of 900 oC and contact time of 3 h was identified as
an ideal condition for the decomposition of raw eggshell powder based on FTIR
and XRD analyses. Both results showed that CaO corresponded to the wavelength
spectrum and diffraction analysis of the sample.
Keywords: calcium carbonate, calcium oxide, eggshell,
calcination
Abstrak
Dalam
konteks ekonomi kitaran, terdapat peluang untuk mengitar semula kulit telur
yang biasanya dibuang di tempat pembuangan sampah. Sebatian utama dalam kulit
telur ialah kalsium karbonat dan ia boleh diuraikan kepada kalsium oksida
melalui proses pengkalsinan. Kajian ini dilakukan untuk mengkaji keadaan
kalsinasi (suhu dan masa pembakaran) yang sesuai bagi kadar penguraian kalsium
karbonat yang optimum. Sampel kulit telur diproses terlebih dahulu untuk
membuang kotoran dan sisa biologi yang tidak diperlukan dan seterusnya dikisar
menjadi serbuk serta diayak. Ciri-ciri fizikal dan kimia utama serbuk kulit
telur seperti perubahan warna, kehilangan jisim, ketumpatan, kandungan lembapan,
pH, sifat terma, dan mengenal pasti ikatan kimia dan sebatian dalam molekul
telah dikaji. Untuk mengkaji ciri-ciri fizikal dan kimia sintesis kalsium
karbonat daripada kulit telur, ujian kandungan lembapan, ketumpatan pukal, pH,
FTIR, and XRD dilaksanakan. Daripada pemerhatian fizikal, hasil menunjukkan
terdapat perbezaan dalam perubahan warna sampel pada pelbagai suhu dan masa
pembakaran. Analisis TGA menunjukkan serbuk kulit telur terurai pada julat suhu
600 oC hingga 900 oC. Hasil FTIR melaporkan bahawa warna kelabu sampel terkalsin
terdiri daripada kalsium karbonat sementara serbuk putih terdiri daripada
kandungan oksida logam. Terdapat tujuh puncak difraksi yang serupa yang
dilaporkan dalam analisis XRD untuk kalsinasi pada suhu 900 oC dan kalsium oksida
(32.25, 37.41, 53.92, 64.18, 67.41, 79.70, dan 88.58). Pengkalsinan serbuk
kulit telur pada suhu 900 oC selama 3 jam dikenal pasti sebagai keadaan yang
sesuai untuk penguraian serbuk kulit telur mentah berdasarkan analisis FTIR dan
XRD. Kedua-dua hasil menunjukkan terdapat kalsium oksida berdasarkan spektrum
gelombang dan analisis difraksi sampel.
Kata kunci: kalsium karbonat, kalsium oksida, kulit telur, kalsinasi
Graphical Abstract
References
1.
Miranda, J.M., Anton, X.,
Redondo-Valbuena, C., Roca-Saavedra, P., Rodriguez, J.A. and Lamas, A. (2015).
Egg and egg-derived foods: Effects on human health and use as functional foods.
Nutrients, 7: 706-729.
2.
Godbert, S.R., Guyot, N. and Nys, Y.
(2019). The golden egg: Nutritional value, bioactivities, and emerging benefits
for human health. Nutrients, 11(684): 1-26.
3.
Laca, A., Paredes, B., Rendueles, M. and
D az, M. (2014). Egg yolk granules: Separation, Characteristics, and
Applications in The Food Industry. LWT - Food Science and Technology,
59(1): 1-5.
4.
Rivera, E.M., Araiza, M., Brostow, W.,
Castano, V.M., Diaz-Estrada, J.R., Hernandez, R. and Rodriguez, J.R. (1999).
Synthesis of hydroxyapatite from eggshells. Materials Letter, 41:
128-134.
5.
Park, S., Choi, K.S., Lee, D., Kim, D.,
Lim, K.T., Lee, K.H., Seonwoo, H. and Kim. J. (2016). Eggshell membrane: Review
and impact on engineering. Biosystem Engineering, 151: 446-463.
6.
De Angelis, G., Medeghini, L., Conte, A.M.
and Mignardi, S. (2017). Recycling of eggshell waste into low-cost adsorbents
for Ni removal from wastewater. Journal of Cleaner Production, 164:
1497-1506.
7.
Carvalho, J., Araujo, J. and Castro, F.
(2011). Alternative low-cost adsorbent for water and wastewater decontamination
derived from eggshell waste: an overview. Waste Biomass Valor, 2:
157-167.
8.
Laca, A., Laca, A. and D az, M. (2017).
Eggshell waste as catalyst: A review. Journal of Environmental Management,
197: 351-359.
9.
Quina, M., Soares, M.A. and Quinta, F.R.
(2017). Applications of industrial eggshells as a valuable anthropogenic
resource. Resources Conservation and Recycling, 123: 176-186.
10.
Ummartyotin S. and Manuspiya, H. (2018). A
critical review of eggshell waste: An effective source of hydroxyapatite as
photocatalyst. Journal of Metals, Materials, and Minerals, 28(1):
124-135.
11.
Sonenklar, C. (1999). Famous for Egg
Waste. The PennState news. The Pennsylvania State University.
https://news.psu.edu/story/140891/1999/09/01/research/famous-egg-waste. [Access
online 1 September 1999]
12.
Arabhosseini, A. and Faridi, H.
(2018). Application of eggshell wastes
as valuable and utilizable products: A review. Resource Agriculture
Engineering, 64: 104-114.
13.
Tangboriboon, N., Kunanuruksapong, R. and
Sirivat, A. (2012). Preparation and properties of calcium oxide from eggshells
via calcination. Materials Science-Poland, 30(4): 313-322.
14.
Ok, Y.S., Lee, S.S., Jeon, WT., Oh, S.E.,
Usman, A.R.A. and Moon, D.H. (2011). Application of eggshell waste for the
immobilization of cadmium and lead in contaminated soil. Environ Geochem
Health 33: 31-39.
15.
Yasar, F. (2019). Biodiesel production via
waste eggshell as a low-cost heterogeneous catalyst: Its effects on some
critical fuel properties and comparison with CaO. Fuel, 255(1): 115828 .
16.
Bashir, A.S.M. and Manusamy, Y. (2015).
Characterization of raw eggshell powder (ESP) as a good bio filler. Journal
of Engineering Research and Technology, 2(1): 56-60.
17.
Ahmad, R., Rohim, R. and Ibrahim, N.
(2015). Properties of waste eggshell as calcium oxide catalyst Applied
Mechanics and Materials, 754: 171-175.
18.
Karthick, J., Jeyanthi, R. and
Petchiyammal, M. (2014). Experimental study on usage of egg shell as partial
replacement for sand in concrete. International Journal of Advanced Research
in Education Technology, 1(1): 7-10.
19.
Gabol, N.A., Memon, F.A., Jawaduddin, M.
and Zardari, Z.H. (2019). Analysis of eggshell powder as a partial replacing
material in concrete. International Journal of Modern Research in
Engineering & Management, 2(9): 22-31.
20.
Razali, N., Azizan, M.A., Pa'ee, K.F.,
Razali, N. and Jumadi, N. (2020). Preliminary studies on calcinated chicken
eggshells as fine aggregates replacement in conventional concrete. Materials
Today: Proceedings, 31(1): 354-359.
21.
Abdulrahman, I., Tijani, H.I., Mohammed,
B.A., Saidu, H., Yusuf, H., Jibrin, M.N. and Mohammed, S. (2014). From garbage
to biomaterials: An overview on eggshell-based hydroxyapatite. Journal of
Materials, 2014: 1-6.
22.
Bartter, J., Diffey, H., Yeung, Y.H.,
O'Leary, F., H sler, B., Maulaga, W. and Alders, R. (2018). Use of chicken
eggshell to improve dietary calcium intake in rural sub-saharan Africa. Maternal
& Child Nutrition, 14(3): 1-10.
23.
Hitchcock, R.K.
(2012). Ostrich eggshell jewelry manufacturing and use of ostrich products
among San and Bakgalagadi in the Kalahari. Botswana Notes and Records,
44: 93-105.
24.
Zoran. A. (2018). The ostrich eggshell
beads craft of the Ju/ hoansi: A reflection on modern craft theories. Craft
Research, 9(2): 229-253.
25.
Abdullah, M., Soo, K. Y., Raofuddin,
D.N.A, Sukor, M.Z., Roslan, A., Ilyas, S.M.M. and Yasin, M.H. (2018). An
evaluation of eggshell waste/waste paper mechanical properties as composite
paper. International Journal of Engineering & Technology, 7:
239-241.
26.
Sulaiman S., and Talha N.S. (2018)
Technique to produce catalyst from egg shell and coconut waste for biodiesel
production. In: Amid A., Sulaiman S., Jimat D., Azmin N. (eds) Multifaceted Protocol in Biotechnology.
27.
Mosaddegh, E. and Hassankhani, A. (2014).
Preparation and characterization of nano-cao based on eggshell waste: Novel and
green catalytic approach to a highly efficient synthesis of pyrano[4,3-b]
pyrans. Chinese Journal Catalyst, 35: 351-356.
28.
Sacia, E.R., Ramkumar, S., Phalak, N. and
Fan, L.S. (2013). Synthesis and regeneration of sustainable CaO sorbents from
chicken eggshells for enhanced carbon dioxide capture. ACS Sustainable
Chemical Engineering, 1: 903-909.
29.
Beck, K., Brunetaud, X., Mertz, J.D. and
Al-Mukhtar, M. (2010). The use of eggshell lime and tuffeau powder to formulate
an appropriate mortar for restoration purposes. Geological Society London
Special Publications, 331(1): 137-145.
30.
Jirimali, H.D., Chaudhari, B.C.,
Khanderay, J.C., Joshi, S.A., Singh, V., Patil, A.M. and Gite, V.V. (2018).
Waste eggshell-derived calcium oxide and nanohydroxyapatite biomaterials for
the preparation of LLDPE polymer nanocomposite and their thermomechanical
study. Polym-Plast Technology Engineering, 57: 804-811.
31.
Tan, Y.H., Abdullah, M.O.,
Nolasco-Hipolito, C. and Zauzi, N.S.A. (2017). Application of RSM and Taguchi
methods for optimizing the transesterification of waste cooking oil catalyzed
by a solid ostrich and chicken-eggshell derived CaO. Renew Energy,
114(Part B): 437-447.
32.
Nagabhushana, K.R., Lokesha, H.S., Reddy,
S.S., Prakash, D., Veerabhadraswamy, M., Bhagyalakshmi, H. and Jayaramaiah,
J.R. (2017). Thermoluminescence properties of CaO powder obtained from chicken
eggshells. Radiation Physics Chemistry, 138: 54-59.
33.
Britannica, T. Editors of Encyclopedia.
Calcination. Encyclopedia Britannica. Retrieved Oct 04, 2016, from
https://www.britannica.com/technology/calcination. [Access online 4 October
2016].
34.
Lin. S., Kiga. T., Wang. Y. and Nakayama.
K. (2011). Energy analysis of CaCO3 calcination with CO2
capture. Energy Procedia, 4: 356-361.
35.
Al-Fatesh, A.S.A. and Fakeeha, A.H.
(2012). Effects of calcination and activation temperature on dry reforming
catalysts. Journal of Saudi Chemical Society, 16: 55-61.
36.
Nordin, N., Hamzah, Z., Hashim, O., Kasim,
F.H., and Abdullah, R. (2015). Effect of temperature in calcination process of
seashells. Malaysian Journal of Analytical Sciences, 19(1): 65-70.
37.
Dampang, S., Purwanti, E., Destyorini, F.,
Kurniawan, S. B. K., Abdullah, S. R. S. and Imron, M. (2021). Analysis of
optimum temperature and calcination time in the production of CaO using
seashells waste as CaCO3 source. Journal of Ecological
Engineering, 22(5): 221 228.
38.
Aina, S., Plessis, B. D., Mjimba, V. and
Brink, H. (2021). Eggshell valorization: Membrane removal, calcium oxide
synthesis, and biochemical compound recovery towards cleaner productions. Biointerface
Research in Applied Chemistry, 12(5): 5870-5883.
39.
Razali, N., Musa, F. A. M., Jumadi, N. and
Jalani, A. Y. (2021). Production of calcium oxide from eggshell: Study on
calcination temperature, raw weight and contact time. RSU International
Research Conference, 2021: 624-637.
40.
Hussein, A. I., Ab-Ghani, Z., Mat, A. N.
C., Ab-Ghani, N. A., Husein, A. and Rahman, I. A. (2021). Synthesis and
characterization of spherical calcium carbonate nanoparticles derived from
cockle shells. Applied Science, 10: 7170.
41.
Mohamad, S.F.S, Mohamad, S. and Jemaat, Z.
(2016). Study of calcination condition on decomposition of calcium carbonate in
waste cockle shell to calcium oxide using thermal gravimetric analysis. ARPN Journal
of Engineering and Applied Sciences, 11(16): 9917-9921.
42.
Islam, K.N., Bakar, M.Z.B.A., Ali, M.E.,
Hussein, M.Z.B., Noordin, M.M., Loqman, M.Y., Miah, G., Wahid, H. and Hashim,
U. (2013). A novel method for the synthesis of calcium carbonate (aragonite)
nanoparticles from cockle shells. Powder Technology, 235: 70-75.
43.
Ali, M. and Badawy W. Z. (2017).
Utilization of eggshells by-product as a mineral source for fortification of
bread strips. Journal of Food and Dairy Sciences, 8(11): 455-459.
44.
Oulego, P., Laca, A., Calvo, S. and Diaz, M. (2019). Eggshell-supported catalysts for
the advanced oxidation treatment of humic acid polluted wastewaters. Water, 12(100):
1-18.
45.
Yahya, N., Tahir, S.M. and Rosli, N.H.M.
(2019). Green eggshell/polypropylene biocomposite. International Journal of
Recent Technology and Engineering, 8: 2277-3878.
46.
Castro, L.D.S., Baranano, A.G., Pinheiro,
C.J.G.L, Menini, L. and Pinheiro, P.F. (2019). Biodiesel production from cotton
oil using heterogeneous CaO catalysts from eggshells prepared at different
calcination temperatures. Green Process Synthesis, 8: 235-244.
47.
Gatea, A.A., Kouzani, A.Z., Kaynak, A., Khoo,
S.Y., Norton, M. and Gates, W. (2018). Soil bulk density estimation
methods: A review. Pedosphere, 28(4): 581-596.
48.
Jewiarz, M., Wrobel, M., Mudryk, K. and
Szufa, S. (2020). Impact of the drying temperature and grinding technique on
biomass grindability. Energies, 13(3392): 1-22.
49.
Queiros, M.V.A., Bezerra, M.N. and Feitosa, J.P.A. (2017). Composite superabsorbent
hydrogel of acrylic copolymer and eggshell: Effect of biofiller addition. Journal
Brazillian Chemical Society, 2017: 1-9.
50.
Loy, C.W., Matori, K.A., Way F.L., Schmid,
S., Zainuddin, N., Wahab, Z.A., Alassan, Z.N. and Zaid, M.H.M. (2016). Effects
of calcination on the crystallography and nonbiogenic aragonite formation of
ark clamshells under ambient conditions. Advances in Materials Science and
Engineering. 2016: 1-8.
51.
Mohadi, R., Anggraini, K., Riyanti F. and Lesbani,
A. (2016). Preparation of calcium oxide (CaO) from chicken eggshells. Sriwijaya Journal of
Environment, 1(2): 32-35.
52.
Krahenbuhl, M., Etter, B. and Udert, K.M. (2015). Pre-treated magnesite as a
source of low-cost magnesium for producing. Science of The Total
Environment, 542: 1155-1161.